Biochemical Reaction Network Modeling¶
Biochemical reaction network (BRN) modeling is an outgrowth of biochemistry and reaction network modeling that was historically used in chemical and petroleum engineering and more recently in a limited context for biological applications. It is an in silico approach that can predict the chemical structures of the metabolites of one or a mixture of chemicals, generate the metabolic pathways, and interconnect these pathways through metabolites in common.
We have been working to develop an in silico BRN modeling framework that we call BioTRaNS (Biochemical Tool for Reaction Network Simulation). Once validated with experimental data, this methodology can predict and simulate in a quantitative and time-dependent manner the formation and disappearance of all metabolites, including potentially toxic reactive intermediates, such as epoxides. Thus, it can aid in predicting drug metabolism, toxicity, and understanding the modes of action of individual chemicals as well as chemical mixtures.
Modeling Framework Structure¶
The figure below shows the conceptual flow of information through BioTRaNS. A Chemical Mixture (concentration of a single or multiple chemicals) and Reaction Environment (types and amounts of enzymes and other agents) are specified by the user. The Chemical Mixture is converted to a set of Virtual Molecules (vMols), each with its own specifications (i.e., a canonical SMILES and list of computed geometric, energetic, and physicochemical properties). SMILES (Simplified Molecular Input Line Entry Specification) is a general-purpose chemical nomenclature and data-exchange format used in computational chemistry. In general, properties are retrieved from associated databases when available, or are computed as needed.
The Reaction Environment is translated into a set of Virtual Enzymes (vEnzs), representing the actual enzymes and Virtual Agents (vAgnts), representing nonenzymatic reactions. Each vEnz is endowed with an appropriate binding calculator and transforms governing the biotransformations that the vEnz can mediate. The binding calculator computes the feasibility of a particular vMol binding to a vEnz, based on quantitative structure-activity relationships (or decision tree) derived from properties of known substrates for each enzyme. Transformations are stored as a list of SMIRKS-based representations. SMIRKS (Smiles Reac[k]tion Specification) is a superset of the SMILES language that describes chemical transformations. Our methodology is unique in implementing transforms that describe steps of a reaction mechanism, including those for enzyme-mediated reactions. The use of mechanistic steps is key to automatically and accurately predicting metabolites (vide infra for an example). After the vMol is created, the binding calculator for each vEnz assesses binding feasibility; if feasible, the vMol becomes an eligible reactant. All eligible reactants then undergo appropriate virtual biotransformations, creating specific chemical reactions and converting the vMol into one or more metabolites, which also are checked for reaction feasibility. The information on the metabolites and the associated transform/agent interconnecting each substrate-product pair is converted to a Virtual Reaction (vRxn). Thus, each vRxn comprises the vMols and vEnzs from which it was derived, as well as appropriate kinetic properties.
Based on the aggregate information in the vRxns, a reaction network is generated in the form of a mathematical graph, wherein the metabolites constitute the vertices or nodes, and the reactions form the edges. Well-described in discrete mathematics, graphs can be efficiently analyzed using algorithms of computer science; graph theory allows efficient determination of characteristics such as common reactants between various pathways, the shortest path to a given metabolite, and common (or all) routes to given metabolites. These network graphs can be depicted at different levels of detail, from highly detailed, showing steps of a reaction mechanism, to overview, showing only key metabolites. Thus, highly complex pathways can be easily simplified. This ability to delineate the metabolites and visualize where pathways intersect is a key tool in gaining new insight into interrelationships among chemicals and their metabolic pathways.
Simultaneous to the graph creation, kinetic properties in each vRxn are used to create the appropriate reaction rate equations (ordinary differential equations, ODE).
These properties include
rate constants (e.g., Michaelis constant, Km, and maximum velocity, Vmax, for enzyme catalyzed reactions and k for nonenzymatic reactions),
inhibitor constants, Ki, and
modes of inhibition or allosterism.
The total set of rate equations and specified initial conditions forms an initial value problem that is solved by a stiff ODE equation solver for the concentrations of all species as a function of time. Virtual Enzymes and Other Virtual Agents. The constituent transforms for the each virtual enzyme are compiled by carefully culling the literature for data on enzymes known to act on the chemicals and chemical metabolites of interest.

Simulation Results¶
As an example of the utility of BioTRaNS, we show below the biochemical reaction networks for selected individual and mixtures of volatile organic compounds (VOCs). These results illustrate the interlinking of pathways, the generation of reactive intermediates, and the utility of the methodology for the elucidation of chemical and biochemical mechanisms.
To generate the results below, only selected agents (vEnzs and vAgnts) were used in the simulations.
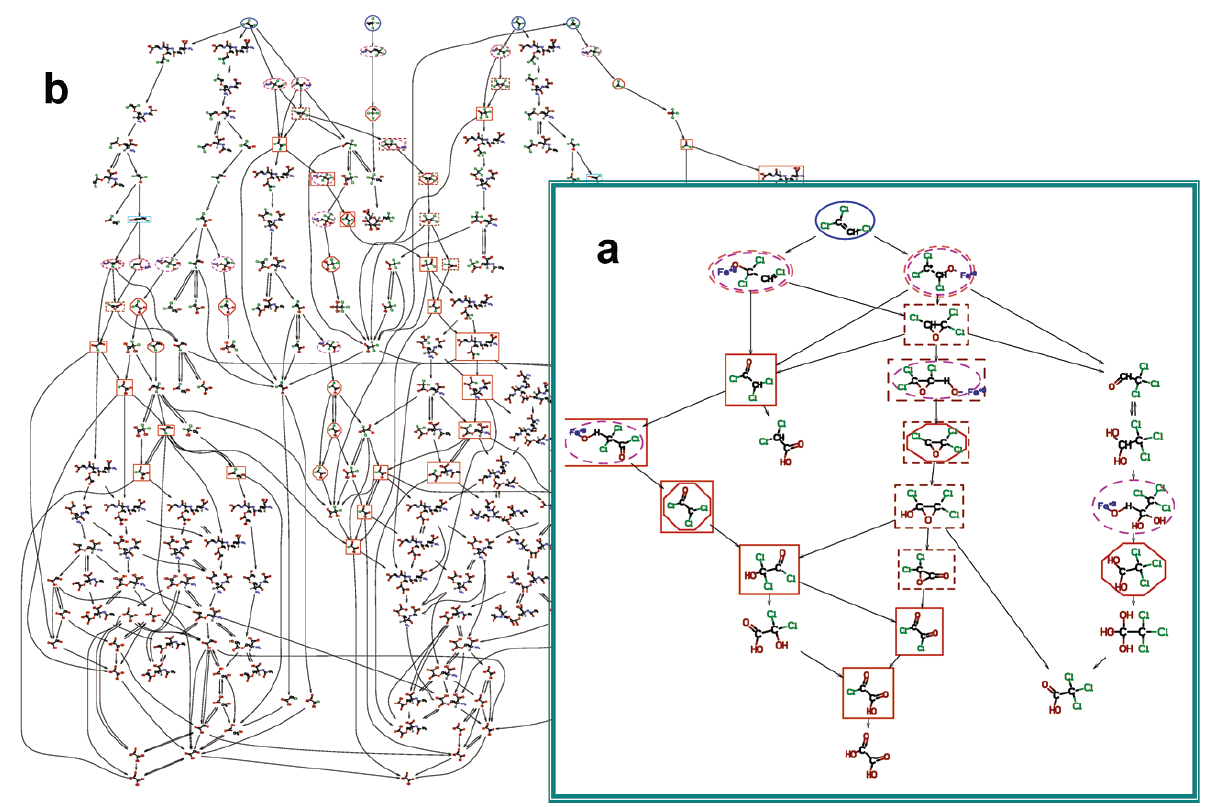
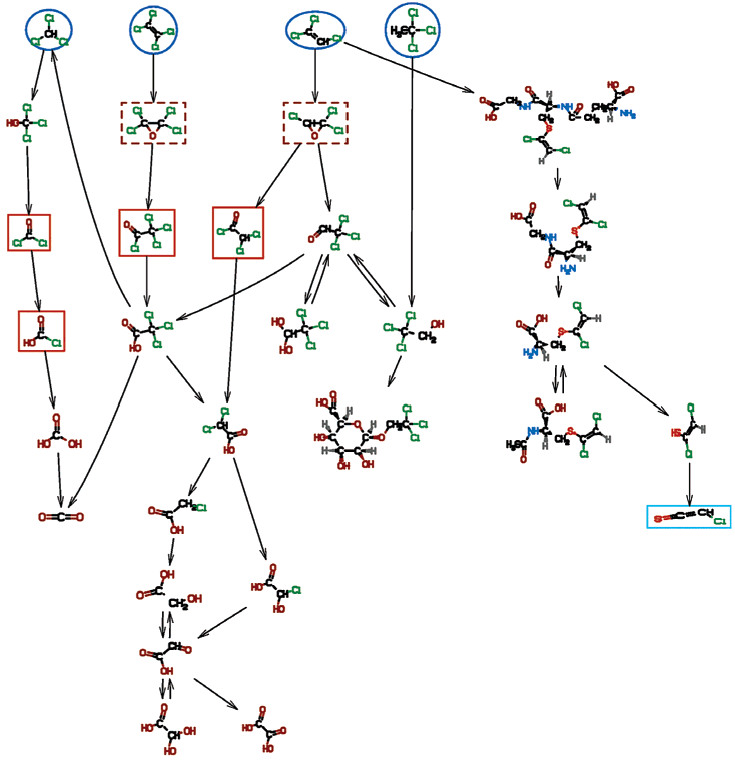
Chemicals are highlighted as follows: starting chemicals (blue, ellipse, solid); epoxides (brown, box, dashed); acid chlorides (orange, box, solid); radicals (red, octagon, solid); Fe-O-substrate complexes (magenta, ellipse, dashed), carbocations (salmon, ellipse, dashed).
BioTRaNS-generated biotransformation pathways for trichloroethylene (TCE):
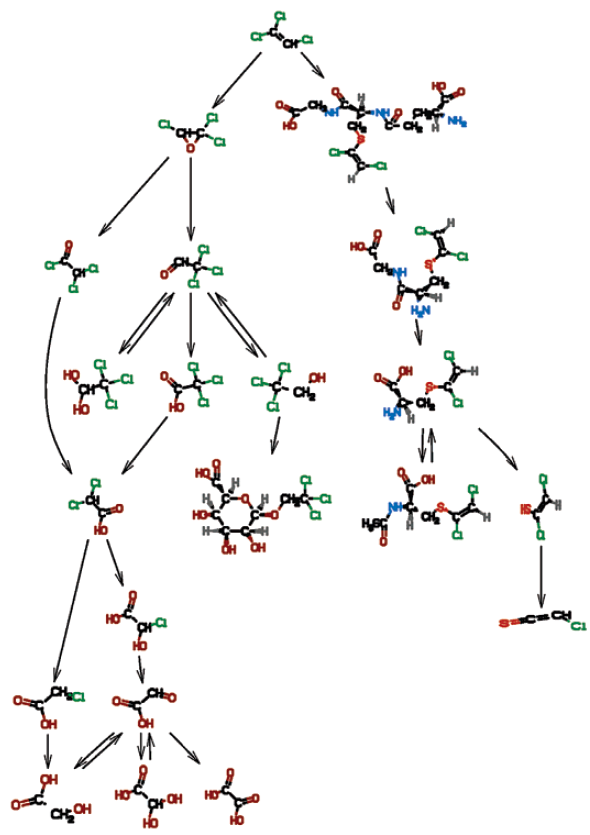
BioTRaNS-generated biotransformation pathways for a mixture of trichloroethylene (TCE), perchloroethylene (Perc), methyl chloroform (MC), and Chloroform (CHCl3):
BioTRaNS-generated mechanism-based pathways for the oxidation of TCE by Cytochrome P450 (a) and a more comprehensive biotransformation pathways of TCE, Perc, MC, and CHCl3 (b):